hardness test of water pdf|water softener hardness chart : retailer of different results and does not necessarily indicate that the hardness is present in the water in this form. 2 This test measures total hardness, ie the total content of calcium and magnesium ions in the water. For the specific measurement of calcium hardness and magnesium hardness, refer to the Palintest Calcium Hardness Test. WEB423K Followers, 1,226 Following, 76 Posts - See Instagram photos and videos from Emily narizinho (@emilynarizinhooficial)
{plog:ftitle_list}
Your customizable and curated collection of the best in trusted news plus coverage of sports, entertainment, money, weather, travel, health and lifestyle, combined with .
Water hardness can be readily determined by titration with the chelating agent EDTA (ethylenediaminetetraacetic acid). This reagent is a weak acid that can lose four protons on .
Water hardness is the traditional measure of the capacity of water to precipitate soap. Hard water requiring a considerable amount of soap to produce leather. Scaling of hot water pipes, boilers .
of different results and does not necessarily indicate that the hardness is present in the water in this form. 2 This test measures total hardness, ie the total content of calcium and magnesium ions in the water. For the specific measurement of calcium hardness and magnesium hardness, refer to the Palintest Calcium Hardness Test. Temporary Hard Water. Temporary hard water is hard water that consists primarily of calcium (Ca 2 +) and bicarbonate (HCO 3-) ions.Heating causes the bicarbonate ion in temporary hard water to decompose into . In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. But in layman's terms, you may notice water hardness when your hands still feel slimy after washing with soap and water, or when your drinking glasses at home become less than crystal clear. Learn a lot more about water hardness on the Water Science .
concentrations. Total water hardness is usually expressed as the milligrams of CaCO3 equivalent to the total amount of calcium and magnesium present in one liter of water (mg/liter, i.e., ppm). Water hardness may range from zero to hundreds of ppm, depending on the source. The classification of degree of water hardness PDF | Investigation of Hardness of Water is very important, so it an effort for water technologist for smooth operation and treatment of water | Find, read and cite all the research you need on . Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall .Collect about 75 cm 3 of soap solution in a small beaker.; Set up a burette and, using the small funnel, fill it with soap solution. Use a measuring cylinder to measure out 10 cm 3 of one of the samples of water from the list below into a conical flask: . Rain water (solution A)
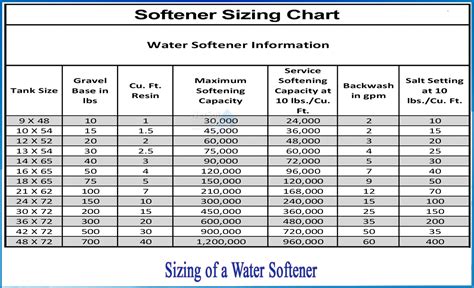
water softener hardness chart
Permanent hardness of water mg/L (CaCO3 Scale) = ml of EDTA used (boiled) *103 /ml of sample Temporary hardness of water mg/L (CaCO3 Scale) = Total hardness of water - Permanent hardness of water Observation: The colour of soluble distilled water and R.O water instantly changed into blue while tap water and pond 6. OBJECTIVE: To determine the hardness, presence of iron, fluoride, chloride depending upon the regional variationin drinking water and study of causes of presence of these ions. INTRODUCTION: Hardness of water is determined by concentration of multivalent cations present in water.Hard water contains Ca2+ , Mg2+ ions.Hardness of water can be removed .Other Learning Activity (6) 174 Experimental Procedures Part A: Determination of total hardness 1. Pipette 50 cm3 mineral water into a conical flask. 2. Add 2 cm3 buffer solution followed by 3 drops of Eriochrome Black T indicator solution. 3. Titrate with 0.01 M EDTA until the solution turns from wine red to sky blue with
water hardness testing near me
Test for total hardness. 5.6.4.1 . Collect a sample of water at the water softener output. 5.6.4.2 . Use an EDTA test or dip-and-read test strips to test for total hardness by measuring the calcium carbonate concentration. 5.6.4.3 . Record the total hardness measured. 5.6.4.4 . If the total hardness is greater than 1 GPG of calcium carbonate .titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. The strips contain EDTA and
Hardness of water - Download as a PDF or view online for free. . Rawalpindi Medical College Follow. This document defines hardness of water as the property of water to form an insoluble curd with soap instead of lather due to the presence of calcium, magnesium, bicarbonates, sulfates and chlorides. It classifies hardness into temporary and .A bathtub faucet with built-up calcification from hard water in Southern Arizona.. Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, [1] which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.. Drinking hard water may .
📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You .2014. This research includes available techniques and simplified methods to estimate and classify the quality of hardness for different water supply sources like sea water, various wells (Fayda, khabyar, jumbyar) and tap water of .The purpose of this experiment is to determine the total hardness of water at the university’s Chemistry Laboratory using a standardized titrant, ethylenediaminetetraacetic acid, by applying the principles of complexometric .
Water Hardness Test Kit, Taylor K-0432, Reagent Pack, Buret, Hardness (calcium/magnesium/total), EDTA, 1 mL = 1 mg CaCO₃This very thoroughly supplied test kit contains ten components and is designed for expert-level testing of the hardness level of potable water. . U.S. Government ATSDR Science Corner - 2.5MB PDF; Turbidity test for .manganese may contribute to water hardness. Water hardness is often defined as the sum of the concentrations of Ca2+ and Mg2+ in water. “Hard” water typically contains high concentrations of Ca2+ and Mg2+, which react with the fatty acids in soap, causing them to precipitate. “Soft” water, such as rainwater or water that has passed . Standard Test Method for Hardness in Water 1 This standard is issued under the Þxed designation D1126; the number immediately following the designation indicates the year of . Hardness Test, mg/L Aluminum, Al +++ 20 5 Ammonium, NH 4 + A 2 000 Bicarbonate, HCO 3 . 500 Bromine, Br . 2 Cadmium, Cd ++ 20 . Carbonate, CO 3 1 000 50 Chloride .
Water Hardness Standard (optional): Orion 923206 Water Hardness Standard, 100 mg/L (ppm). Reagent Solutions: Purchased or prepared ammonia buffer, . ASTM D1126, Standard Test Method for Hardness in Water. ASTM International, West Conshohocken, PA, USA. www.astm.org. 3. ISO 6059-1984, Water Quality – Determination of the sum of calcium and . Some tests, like the Vickers hardness test, can be used on a macro scale as well as a micro scale. The loads required are listed in Table 1 below: Table 1: Different Hardness Test Methods and Their Corresponding Loads. Method Load Range Standard; Method. Brinell. Load Range. 1 kgf–3,000 kgf. Standard. ASTM E10, ISO 6506. Method. A major application of EDTA titration is testing the hardness of water, for which the method described is an official one. Hardness of water also can be tested by a more rapid test strip method. The commercial test strips contain EDTA and an indicator chemical to cause a color change when the calcium and magnesium in water react with the EDTA.
dartec universal testing machine
Water hardness results from calcium and magnesium salts. . More. Store. Talk to our experts. 1800-120-456-456. Sign In. Hardness of Water. JEE Main; Chemistry; Hardness Of Water; Download PDF. Study Material. Important Questions. Chapter Pages. Revision Notes. Formulas. Difference Between . Maths Mock Test. Physics Mock Test. Chemistry Mock .
does not mix well with soaps. Because of this, you can test for the hardness of water by looking at the amount of bubbles produced when you mix water and soap. Aim: To test various solutions of water for hardness and see how effective different filtering materials are at removing the minerals that cause the hardness. A major application of EDTA titration is testing the hardness of water, for which the method described is an official one. Hardness of water also can be tested by a more rapid test strip method. The commercial test strips contain EDTA and an indicator chemical to cause a color change when the calcium and magnesium in water react with the EDTA.monly see on the report. Hard water is a purely aes-thetic problem that causes soap and scaly deposits in plumbing and decreased cleaning action of soaps and detergents. Hard water can also cause scale buildup in hot water heaters and reduce their effective lifetime. Table 4 will help you interpret the hardness parameters cited on your analysis.
testing water hardness at home
webSpankBang is the ultimate destination for free porn videos and 4K sex movies. Enjoy millions of fresh and exclusive scenes featuring the hottest stars and amateurs. Discover the best porn channels, the most popular and trending videos, and the upcoming releases. SpankBang will make you cum like never before!
hardness test of water pdf|water softener hardness chart